Answer:
6.82% is the mass percent NaOH in the solution.
Step-by-step explanation:
Mass of the solute that is NaOH = m = 20 g
Mass of the solution = M
Volume of the solution = V = 255 mL
Density of the solution = d = 1.15 g/mL

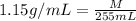
M = 293.25 g
Mass percentage of solute :(w/w)%
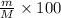
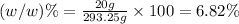
6.82% is the mass percent NaOH in the solution.