Answer:
The weight percent of NaBr is 25,7%
Step-by-step explanation:
Molality is a way to express the concentration of a chemical in terms of moles of substances per kg of solution. Weight percent is other way to express concentration in terms of mass of substance per mass of solution per 100
Thus, to obtain weight percent you must convert moles of NaBr to grams with molar mass and these grams to kilograms, thus:
2,50 mol / kg solution × (102,9 g / 1mol) × (1 kg / 1000 g) =
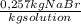 × 100 = 25,7 % (w/w)
× 100 = 25,7 % (w/w)
I hope it helps!