Answer:
Step-by-step explanation:
Given
ambient Pressure =98.10 kPa
(a)gauge pressure 152 kPa
we know
Absolute pressure=gauge pressure+Vacuum Pressure
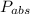 =152+98.10=250.1 kPa or 36.27 psi
=152+98.10=250.1 kPa or 36.27 psi
(b)
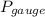 =67.5 Torr or 8.99 kpa
=67.5 Torr or 8.99 kpa
as 1 Torr is 0.133 kPa
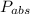 =8.99+98.10=107.09 kPa or 15.53 psi
=8.99+98.10=107.09 kPa or 15.53 psi
(c)
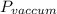 =0.1 bar or 10 kPa
=0.1 bar or 10 kPa
Thus absolute pressure=98.10-10=88.10 kPa or 12.77 psi
as 1 kPa is equal to 0.145 psi
(d)
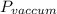 =0.84 atm or 85.113 kPa
=0.84 atm or 85.113 kPa
as 1 atm is equal to 101.325 kPa
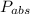 =98.10-85.11=12.99 kPa or 1.88 psi
=98.10-85.11=12.99 kPa or 1.88 psi