Answer:
a) [Tris0] : [Tris] = 1 : 100
b) Range = 7.1 to 9.1
Step-by-step explanation:
a) Calculation of ratio of the basic and the acidic forms of tris
pH of a buffer is calculate using Henderson-Hasselbalch equation
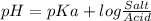
Conjugate acid of Tris dissociated as
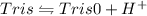
For tris,
Salt or Basic form = tris0
Acid or Acidic form = Tris
pKa = 8.1
pH = 6.1
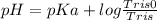
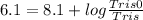
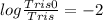
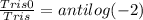
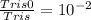
[Tris0] : [Tris] = 1 : 100
b) Range of Tris
Range of any buffer is:
From (pKa -1) to (pKa+1)
So, range of Tris is:
From (8.1 - 1) to (8.1 +1)
or from 7.1 to 9.1