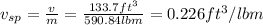First, let's make some convertions:
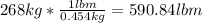
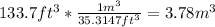
a) weight of the fuel:
Newtons: The weight in newtons is equal to the mass in kilograms times the gravity in m/s^2.
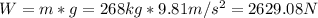
lbf: The weight inlbf is equal to the mass in slugs times the gravity in ft/s^2.
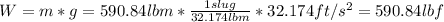
b) density:
The density is the mass in kg of the fuel divided by its volume in m^3:
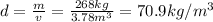
c) specific volume:
The specific volume is the volume in ft^3 of the fuel divided by its mass in lbm: