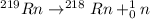Answer: Option (c) is the correct answer.
Step-by-step explanation:
A positron is denoted as
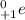 , a neutron is denoted as
, a neutron is denoted as
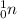 , an electron is denoted as
, an electron is denoted as
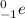 , and a proton is denoted as
, and a proton is denoted as
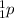 .
.
Therefore, when
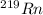 will decay into
will decay into
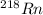 this means there occurs decrease in its mass number. Hence, it means a neutron will be releasing during this reaction.
this means there occurs decrease in its mass number. Hence, it means a neutron will be releasing during this reaction.
The reaction will be as follows.