Answer : The 3D representation of
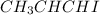 are shown below.
are shown below.
Explanation :
The configuration of the geometrical isomers is designated by two system which are, Cis-trans system and E-Z system.
The rules for E-Z system are :
The atoms or groups attached to each olefinic carbon are given priority as per the sequence rule.
If the higher priority groups are present on same sides across the double bond, the geometrical isomer is said to have Z-configuration or 'cis'.
If the higher priority groups are present on opposite sides across the double bond, the geometrical isomer is said to have E-configuration or 'trans'.
In the given compound, higher priority groups are
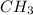 and
and
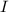 .
.
When
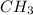 and
and
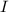 are present on same sides across the double bond, the geometrical isomer is said to have 'cis'.
are present on same sides across the double bond, the geometrical isomer is said to have 'cis'.
When
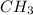 and
and
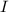 are present on opposite sides across the double bond, the geometrical isomer is said to have 'trans'.
are present on opposite sides across the double bond, the geometrical isomer is said to have 'trans'.
The 3D representation is shown by dash and wedge bonds in which dash shows that the molecules are below the plane and wedge shows that the molecules are above the plane.