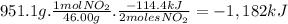Answer:
The amount of released is 1,182 kJ.
Step-by-step explanation:
When heat is released at constant pressure, this change in energy is known as enthalpy (ΔH°) of the reaction. Enthalpy is an extensive property, so it depends on the amount of reacting material. Let's take a look at the provided equation:
2 NO(g) + O₂ ⇄ 2 NO₂(g) ΔH° = -114.4 kJ
Since this equation is balanced with 2 moles of NO₂(g), we can say that 114.4 kJ are released every 2 moles of NO₂(g) produced. By convention, when enthalpies are negative, it means that energy is released and the reaction is exothermic. Conversely, positive enthalpies mean energy is absorbed and the reaction is endothermic.
We can calculate the amount of energy released taking into account the previous relationship (-114.4 kJ/2 moles of NO₂(g)), the mass of NO₂(g) produced (951.1g) and its molar mass (46.00g/mol). The calculations would be: