Answer:
The density of the metal is 21.675 g/mL.
The volume of metal with mass of 64.9 grams is 2.994 mL.
Step-by-step explanation:
Density is defined as mass present in unit volume of the substance.

Mass of unknown metal = m = 427.0 g
Volume of the unknown metal = v = 19.7 mL
Density of the unknown metal = d
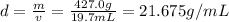
Mass of the same unknown metal = M = 64.9 g
Volume of same unknown metal with mass M = V
Density of the metal = d = 21.675 g/mL
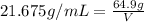
V = 2.994 mL