Step-by-step explanation:
The given data is as follows.
 = 1.5 atm,
= 1.5 atm,
 = 20 L,
= 20 L,
 = (28 + 273) K = 301 K
= (28 + 273) K = 301 K
 = 5 atm,
= 5 atm,
 = ?,
= ?,
 = (50 + 273) K = 323 K
= (50 + 273) K = 323 K
Formula to calculate the volume will be as follows.
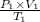 =
=
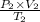
Putting the given values into the above formula as follows.
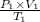 =
=
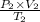
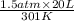 =
=
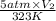
 = 0.64 L
= 0.64 L
Thus, we can conclude that the change in volume of the balloon will be 0.64 L.