Answer:
946.92 kJ
Step-by-step explanation:
This process has 3 parts:
1. The first part, where the temperature of Ethyl alcohol remains constant and it changes from gas to liquid.
2. The second part, where the temperature drops from 78°C to -114°C
3. The third parts, where the temperature remains constant and it changes from liquid to solid.
The energy lost in a phase change is:
Q = m*cl
The energy lost because of the drop in temperature is:
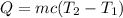
cl is the heat of vaporization or heat of fusion, depending on the type of phase change. c is the specific heat.
So, the energy lost in each part is:
1.
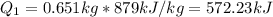
2.
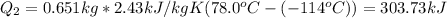
3.
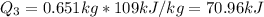
Then, the total energy removed should be:
Q = Q1 + Q2 + Q3 = 572.23 kJ + 303.73kJ + 70.96kJ = 946.92 kJ