Answer: 12.5
Step-by-step explanation:
Molarity of a solution is defined as the number of moles of solute dissolved per Liter of the solution.
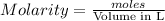
moles of
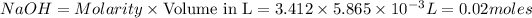
moles of
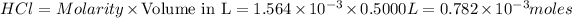

According to stoichiometry:
1 mole of
 require 1 mole of
require 1 mole of
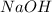
Thus
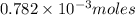 will combine with
will combine with
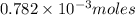 of
of
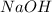
Thus
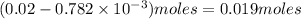 of
of
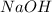 will be left
will be left
Thus Molarity of
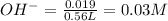
![pOH=-\log[OH^-]](https://img.qammunity.org/2020/formulas/chemistry/high-school/n477c3o3xy8p6ug3ipfjqh9fd53hdrrjyq.png)
Putting in the values:
![pOH=-\log[0.03]](https://img.qammunity.org/2020/formulas/chemistry/college/urhf9elotz4cq1szl2idgwjl5udz6ulrt9.png)
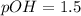
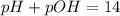
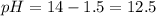
Thus final pH will be 12.5.