Answer: The density of the metal is 10.60 g/mL and the volume occupied by 86.0 grams is 8.11 mL
Step-by-step explanation:
To calculate the density of unknown metal, we use the equation:
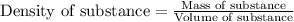 ......(1)
......(1)
Volume of unknown metal = 19.8 mL
Mass of unknown metal = 210.0 g
Putting values in equation 1, we get:
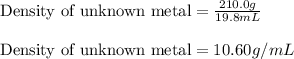
The density of the metal remains the same.
Now, calculating the volume of unknown metal, using equation 1, we get:
Density of unknown metal = 11.45 /mL
Mass of unknown metal = 86.0 g
Putting values in above equation, we get:
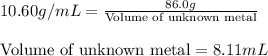
Hence, the density of the metal is 10.60 g/mL and the volume occupied by 86.0 grams is 8.11 mL