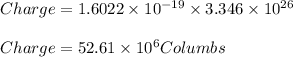Answer:
Part 1) Number of electrons in 1 liter of water equals
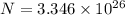
Part 2) Net charge of all the electrons equals
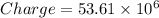
Step-by-step explanation:
Since we know that the density of water is 1 kilogram per liter thus we infer that mass of 1 liter of water is 1 kilogram hence we need to find electron's in 1 kg of water.
Now since it is given that molar mass of water is 18.0 grams this means that 1 mole of water contains 18 grams of water.
Hence by ratio and proportion number of moles in 1 kg water equals
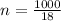
Now by definition of mole we know that 1 mole of any substance is Avagadro Number of particles.
Hence the no of molecules in 'n' moles of water equals
![n'=(1000)/(18)\cdot N_a\\\\n'=(1000)/(18)\cdot 6.023* 10^(23)\\[\tex][tex]\\n'=3.346* 10^(25)](https://img.qammunity.org/2020/formulas/physics/college/cezz2i6iuxmkhtcst8pqifisuauqnep1rq.png)
Now since it is given that each molecule has 10 electron's thus the total number of electrons in n' molecules equals
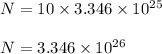
Part 2)
We know that charge of 1 electron equals
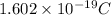 the the charge of electrons in 'N' quantity equals
the the charge of electrons in 'N' quantity equals