Step-by-step explanation:
The given data is as follows.
Volume = 1 L, Concentration of Ca = 5 ppm or 5 mg/L
As 1 mg = 0.001 g so, 5 mg /L will be equal to 0.005 g/l. Molar mass of calcium is 40.078 g/mol.
Hence, calculate molarity of calcium as follows.
Molarity of Ca =
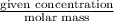
=
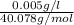
Molarity of Ca =
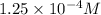
Hence, molarity of
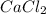 is
is
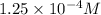 . Since, volume is same so, moles of calcium chloride will be
. Since, volume is same so, moles of calcium chloride will be
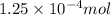 .
.
Thus, we can conclude that mass of
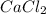 will be as follows.
will be as follows.
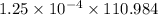 (molar mass of
(molar mass of
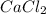 = 110.984 g/mol)
= 110.984 g/mol)
= 0.0138 g
Thus, we can conclude that mass of
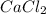 is 0.0138 g.
is 0.0138 g.