Answer: D) 936 K
Step-by-step explanation:
Given:
Initial temperature of the gas,
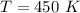
Initial Pressure of the gas,
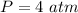
initial volume of the gas,
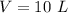
It it given that the process is adiabatic, so for a adiabatic process we have
Let
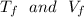 be the final temperature and volume of the gas.
be the final temperature and volume of the gas.
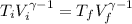
For monotomic gas
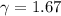
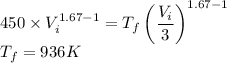
Hence the final temperature of the gas is 936 K. So option D is correct