Step-by-step explanation:
It is given that molarity is 0.15 M and volume is 250.0 ml. As 1 ml equals 0.001 l.
Hence, 250.0 ml = 0.25 l.
Also, molarity is equal to the number of moles present in liter of solution. Therefore, calculate number of moles as follows.
Molarity =
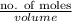
0.15 M =

No. of moles = 0.0375 mol
Thus, we can conclude that moles present in given solution are 0.0375.