Answer:
46.004 g/mol
Step-by-step explanation:
Scottish physicist Thomas Graham formulated a law known as Graham's law of effusion in 1848. He conducted an experiment and found the relationship between the rate of effusion of a gas and its molar mass as:
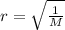
where,
r is the rate of effusion of a gas
M is the molar mass of the gas.
Also, r = v/t
And for two gases taking different t₁ and t₂ to effuse, the formula is:
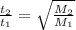
So,
For Neon :
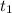 = 8.61 minutes
= 8.61 minutes
 = 20.1797 g/mol
= 20.1797 g/mol
For unkown gas:
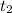 = 13.0 minutes
= 13.0 minutes
 = ? g/mol
= ? g/mol
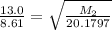
Molar mass of unknown gas = 46.004 g/mol