Answer:
a) pH = 2,23
b) pH = 3,26
c) pH = 3,74
d) pH = 7,98. Here we have the equivalence point of the titration
Step-by-step explanation:
In a titration of a strong base (NaOH) with a weak acid (HCOOH) the reaction is:
HCOOH + NaOH → HCOONa + H₂O
a) Here you have just HCOOH, thus:
HCOOH ⇄ HCOO⁻ + H⁺ where ka =1,8x10⁻⁴ and pka = 3,74
When this reaction is in equilibrium:
[HCOOH] = 0,200 -x
[HCOO⁻] = x
[H⁺] = x
Thus, equilibrium equation is:
1,8x10⁻⁴ =
![([x][x] )/([0,200-x])](https://img.qammunity.org/2020/formulas/chemistry/college/vl8erizjp88kiipsa56l2b199o4a450p7d.png)
The equation you will obtain is:
x² + 1,8x10⁻⁴x - 3,6x10⁻⁵ = 0
Solving:
x = -0,006090675 ⇒ No physical sense. There are not negative concentrations
x = 0,005910674
As x = [H⁺] and pH = - log [H⁺]
pH = 2,23
b) Here, it is possible to use:
HCOOH + NaOH → HCOONa + H₂O
With adition of 5,00 mL of 1,00M NaOH solution the initial moles are:
HCOOH =
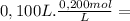 = 2,0x10⁻² mol
= 2,0x10⁻² mol
NaOH =
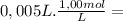 = 5,0x10⁻³ mol
= 5,0x10⁻³ mol
HCOO⁻ = 0.
In equilibrium:
HCOOH = 2,0x10⁻² mol - 5,0x10⁻³ mol = 1,5x10⁻² mol
NaOH = 0 mol
HCOO⁻ = 5,0x10⁻³ mol
Now, you can use Henderson–Hasselbalch equation:
pH = 3,74 + log

pH = 3,26
c) With adition of 10 mL of 1,00M NaOH solution the initial moles are:
HCOOH =
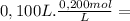 = 2,0x10⁻² mol
= 2,0x10⁻² mol
NaOH =
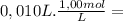 = 1,0x10⁻² mol
= 1,0x10⁻² mol
HCOO⁻ = 0.
In equilibrium:
HCOOH = 2,0x10⁻² mol - 1,0x10⁻² mol = 1,0x10⁻² mol
NaOH = 0 mol
HCOO⁻ = 1,0x10⁻² mol
Now, you can use Henderson–Hasselbalch equation:
pH = 3,74 + log

pH = 3,74
d) With adition of 20 mL of 1,00M NaOH solution the initial moles are:
HCOOH =
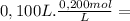 = 2,0x10⁻² mol
= 2,0x10⁻² mol
NaOH =
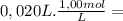 = 2,0x10⁻² mol
= 2,0x10⁻² mol
HCOO⁻ = 0.
Here we have the equivalence point of the titration, thus, the equilibrium is:
HCOO⁻ + H₂O ⇄ HCOOH + OH⁻ kb = kw/ka where kw is equilibrium constant of water = 1,0x10⁻¹⁴; kb = 5,56x10⁻¹¹
Concentrations is equilibrium are:
[HCOOH] = x
[HCOO⁻] = 0,1667-x
[OH⁻] = x
Thus, equilibrium equation is:
5,56x10⁻¹¹ =
![([x][x] )/([0,01667-x])](https://img.qammunity.org/2020/formulas/chemistry/college/5cle4msae7q1epckbr8njp52lq7wtxw1jc.png)
The equation you will obtain is:
x² + 5,56x10⁻¹¹x - 9,27x10⁻¹³ = 0
Solving:
x = -9,628361x10⁻⁷⇒ No physical sense. There are not negative concentrations
x = 9,627806x10⁻⁷
As x = [OH⁻] and pOH = - log [OH⁻]; pH = 14 - pOH
pOH = 6,02
pH = 7,98
I hope it helps!