Answer: The number of electrons for n = 0, 1 and 2 are 2, 6 and 10 respectively.
Step-by-step explanation:
Huckel's rule is used to determine the aromaticity in a compound. The number of delocalized
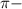 electrons are calculated by using the equation:
electrons are calculated by using the equation:
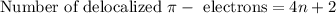
where,
n = 0 or any whole number
- Calculating the value of electrons for n = 0
Putting values in above equation, we get:

- Calculating the value of electrons for n = 1
Putting values in above equation, we get:

- Calculating the value of electrons for n = 2
Putting values in above equation, we get:

Hence, the number of electrons for n = 0, 1 and 2 are 2, 6 and 10 respectively.