Answer: Yes,
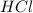 is a strong acid.
is a strong acid.
acid =
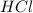 , conjugate base =
, conjugate base =
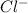 , base =
, base =
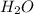 , conjugate acid =
, conjugate acid =
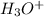
Step-by-step explanation:
According to the Bronsted-Lowry conjugate acid-base theory, an acid is defined as a substance which looses donates protons and thus forming conjugate base and a base is defined as a substance which accepts protons and thus forming conjugate acid.
Yes
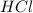 is a strong acid as it completely dissociates in water to give
is a strong acid as it completely dissociates in water to give
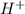 ions.
ions.
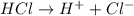
For the given chemical equation:
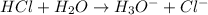
Here,
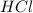 is loosing a proton, thus it is considered as an acid and after losing a proton, it forms
is loosing a proton, thus it is considered as an acid and after losing a proton, it forms
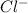 which is a conjugate base.
which is a conjugate base.
And,
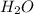 is gaining a proton, thus it is considered as a base and after gaining a proton, it forms
is gaining a proton, thus it is considered as a base and after gaining a proton, it forms
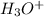 which is a conjugate acid.
which is a conjugate acid.
Thus acid =
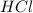
conjugate base =
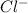
base =
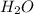
conjugate acid =
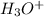 .
.