Step-by-step explanation:
According to the Le Chatelier's principle, any disturbance caused in an equilibrium reaction will shift the direction of equilibrium where there is less stress or disturbance.
For example,
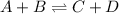
So, when we increase the concentration of D in this reaction then disturbance will increase on the product side.
And, frequency of successful collisions between the reactant atoms will decrease. Hence, there will be less stress on reactant side.
As a result, equilibrium will shift in the backward direction that is, on the reactant side it will shift.