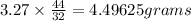Answer:
Amount of Carbon dioxide equals 4.49625 grams.
Step-by-step explanation:
From the basic stichometric reaction between carbon and oxygen we know that 1 mole of carbon combines with 1 mole of oxygen to form 1 mole of carbon dioxide.
Thus we can say that 12 grams of carbon combines with 32 grams of oxygen to form 44 grams of carbon dioxide.
In the given question assuming that there is no limited supply of carbon we can find the find the amount of carbon dioxide formed from 3.27 grams of Oxygen using ratio and proportion method.
As we can see that 32 grams of oxygen form 44 grams of carbon dioxide thus we can say 1 gram of oxygen yields
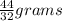 Carbon Dioxide
Carbon Dioxide
Thus the carbon dioxide formed by 3.27 grams of Oxygen equals