Answer: a) 0.08157 moles of
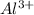
b) 0.24471 moles of
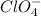
c) 0.97884 moles of oxygen atoms
Explanation:-
The dissociation of the given compound is shown by the balanced equation:
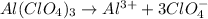
According to stoichiometry:
a) 1 mole of
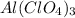 produces 1 mole of
produces 1 mole of
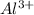
Thus 0.08157 mole of
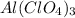 produces=
produces=
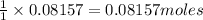 of
of
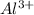
b) 1 mole of
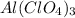 produces 3 moles of
produces 3 moles of
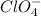
Thus 0.08157 mole of
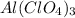 produces=
produces=
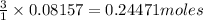 of
of
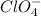
c) 1 mole of
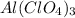 produces 12 moles of oxygen atoms
produces 12 moles of oxygen atoms
Thus 0.08157 mole of
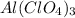 produces=
produces=
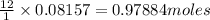 of oxygen atoms
of oxygen atoms