Step-by-step explanation:
It is given that,
Initial state of electron,

Final state of electron,

The wavelength of the excited electron is given by :

Where
R is Rydberg's constant


or

Let f is the frequency of the observed photon. It is given by :
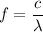


Hence, this is the required solution.