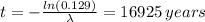Answer:
The bones are 16925 years old
Step-by-step explanation:
We have to use the radioactive decay law and know that the half life of carbon-14 is
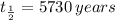 . From this information we can know the decay rate of the carbon 14,
. From this information we can know the decay rate of the carbon 14,
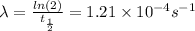
Now to know the age of the bones we must directly use the radioactive decay law:
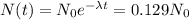
Where the rightmost part of the equation comes from the statement that the activity found is just 12.9% of the activity that would be found in a similar live animal. This means that the number of carbon-14 atoms is just 12.9% of what it was at the moment the saber-toothed tiger died.
Solving for t we have: