Answer:
The energy of each photon is
 Joule.
Joule.
Explanation:
Consider the provided information.
According to the plank equation:
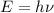
Where E is the energy of photon, h is the plank constant and
 is the frequency.
is the frequency.
It is given that
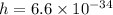 and
and
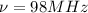
98Mhz =
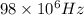
Substitute the respective value in plank equation.
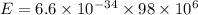
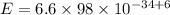
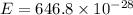
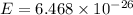
Hence, the energy of each photon is
 Joule.
Joule.