Answer:
466mEq
Explanation:
First, we need to know the concentration of NaCl in a normal saline solution, this is by definition 0.9%, meaning we have 0.9g of NaCl per 100ml of solution, we want to know how much NaCl we have in 3L (3000ml):
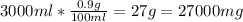
So, we have 27000mg in 3L of normal saline solution.
Now, acording to our milliequivalent (mEq) equation (
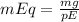 ) where pE is de molecular mass of NaCl divided by their charges, in this case 1:
) where pE is de molecular mass of NaCl divided by their charges, in this case 1:
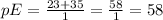
Finally we substitute in the mEq formula:
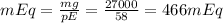
I hope you find this information useful! Good luck!