Answer:
238.25 mg
Explanation:
Given:
Molar mass of MgCl₂ = 95.3
atomic weight of Mg₂ = 243
Atomic weight of Cl = 35.5
Volume of solution required = 5.0 mEq of magnesium
Now,
mEq =
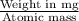
on substituting the values, we get
5 =
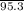
or
weight of magnesium chloride = 238.25 mg
Therefore,
the required mass of MgCl₂ is 238.25 mg