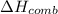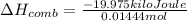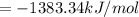Answer:
1383.34 kJ/mol is the energy released on combustion of the organic compound.
Step-by-step explanation:
Mass of an organic compound = 0.6654 g
Molar mass of organic compound = 46.07 g/mol
Moles of an organic compound =
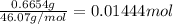
Let heat evolved during burning of 0.6654 grams of an organic compound be -Q.
Heat absorbed by calorimeter = Q' = -Q
The total heat capacity of the calorimeter all its contents = C
C = 3576 J/°C
Change in temperature of the calorimeter =
ΔT = 30.589°C - 25.000°C = 5.589°C
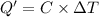
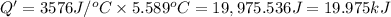
Q' = 19.975 kJ
Q = -19.975 kJ (negative sign; energy released)
0.01444 moles of an organic compound gives 19.975 kilo Joule.
The 1 mole of an organic compound will give :