Answer : The correct option is, (C) NH₃ + H⁺
Step-by-step explanation:
Hydrolysis : It is defined as the chemical reaction in which the breakdown of compound takes place due to reaction with water.
As per question:
First ammonium chloride completely dissociates into ion.
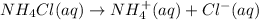
Now ammonium ion react with water to give ammonia and hydronium or hydrogen ion.
The balanced hydrolysis reaction will be:
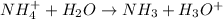
Hence, the correct option is, (C) NH₃ + H⁺