Answer:
The attraction force between the cation and anion is
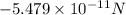
Solution:
According to the question:
Separation distance between the center of the charges, d = 2.9 nm =
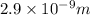
Also, valence is the no. of electrons required to reach the stable configuration of octet in the outer most orbit or shell of an atom.
Thus
+ 2 means +2e = Q
-1 means - 1e = Q'
where
e = electronic charge =
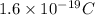
The Coulombian Force is given as:
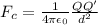
where
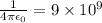
Now,
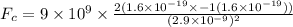
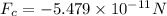
Here, the negative sign is indicative of the attractive nature of force between two oppositely charged particles.