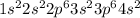Answer:
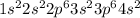
Step-by-step explanation:
Calcium is the chemical element with symbol Ca and the atomic number equal to 20. As alkaline earth metal, the element, calcium is reactive metal. IT lies in the second group and forth period of the periodic table.
The number of the valence electrons of the calcium element is 2 and thus primarily denotes these electrons and forms ionic bond.
The ground state- electron configuration for calcium is: