Step-by-step explanation:
According to the ideal gas equation, PV = nRT.
where, P = pressure, V = volume
n = no. of moles, R = gas constant
T = temperature
Also, density is equal to mass divided by volume. And, no. of moles equals mass divided by molar mass.
Therefore, then formula for ideal gas could also be as follows.
P =
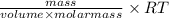
or, P =
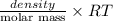
Since, density is given as 0.789 g/ml which is also equal to 789 g/L (as 1000 mL = 1 L). Hence, putting the given values into the above formula as follows.
P =
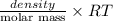
=
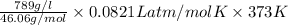
= 525 atm
As two-liter soft drink bottle can withstand a pressure of 5 atm and the value of calculated pressure is 525 atm which is much greater than 5 atm.
Therefore, the soft drink bottle will obviously explode.