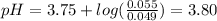Answer:
pH of solution is 3.80
Step-by-step explanation:
- Formic acid an weak acid and formate is conjugate base of formic acid
- Hence solution containing formic acid and formate acts as a buffer.
- According to Henderson-Hasselbalch equation for a buffer consist of an weak acid (formic acid) and it's conjugate base (formate)-
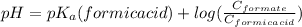
where, C stands for concentration
 of formic acid 3.75
of formic acid 3.75
So,