Answer:

Step-by-step explanation:
Let r is the radius of the n = 1 orbit of the gold. According to Bohr's model, the radius of orbit is given by :
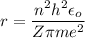
Where
n = number of orbit
h = Planck's constant
Z = atomic number (for gold, Z = 79)
m = mass of electron
e = charge on electron
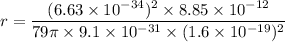

So, the radius of the n = 1 orbit of gold is
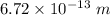 . Hence, this is the required solution.
. Hence, this is the required solution.