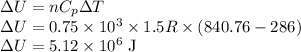Answer:
Work done,
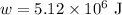
change in internal Energy ,
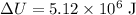
Step-by-step explanation:
Given:
- Mass of helium gas
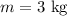
- initial temperature
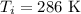
Since It is given that the process is adiabatic process it means that there is no exchange of heat between the system and surroundings
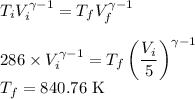
Let n be the number of moles of Helium given by
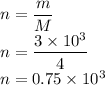
Work done in Adiabatic process
Let W be the work done
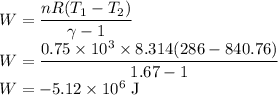
The Internal Energy change in any Process is given by
Let
 be the change in internal Energy
be the change in internal Energy