Answer :
(a). The wavelength of electron is 26.22 μm.
(b).The wavelength of car is
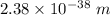
Explanation :
Given that,
Speed = 100 km/hr
Mass of car = 1 ton
(a). We need to calculate the wavelength of electron
Using formula of wavelength
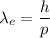
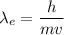
Put the value into the formula
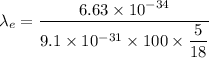
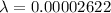
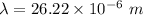
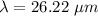
(II). We need to calculate the wavelength of car
Using formula of wavelength again
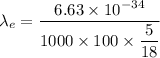
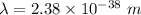
The wavelength of the electron is greater than the dimension of electron and the wavelength of car is less than the dimension of car.
Therefore, electron is quantum particle and car is classical.
Hence, (a). The wavelength of electron is 26.22 μm.
(b).The wavelength of car is
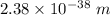 .
.