Step-by-step explanation:
Given that,
Mass of gas = 222 g
Temperature = 54.43°
Pressure = 4.45 atm
Final volume = 2.25 initial volume
For isothermal expansion
(a). We need to calculate the pressure
Using relation of pressure and volume
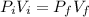
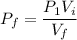
Put the value into the formula
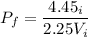
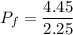
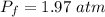
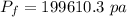
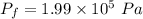
The pressure is
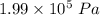
(b). We need to calculate the work done
1 mole of Hg is 200.59 gram
222 g of Hg is
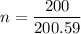
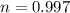
Using formula of work done
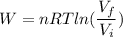
Put the value into the formula
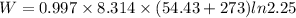
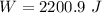
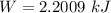
The work done is 2.20 kJ.
(c). We need to calculate the gas absorb
Heat absorbed by the gas is the work done
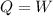
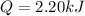
The absorb heat is 2.20 kJ.
(d). We need to calculate the change in the total internal energy of the gas
Change in internal energy in an isothermal process is zero.
So,
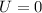
Hence, This is the required solution.