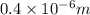Answer:
The longest wavelength equals
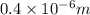
Step-by-step explanation:
According to Einstein's photoelectric equation we have
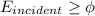
where
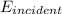 is the energy of the incident light
is the energy of the incident light
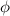 is the work function of the metal
is the work function of the metal
The incident energy of the light with wavelength
 is given by
is given by
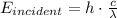
Thus the photoelectric equation reduces to
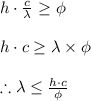
Thus applying values we get
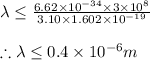
Hence The longest wavelength equals