Answer:
The speed is 33.5 m/s.
Step-by-step explanation:
Given that,
Mass = 0.064 kg
Wavelength
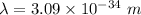
We need to calculate the speed
Using formula of he de Broglie wavelength
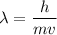
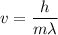
Where, h = Planck constant
m = mass
 = wavelength
= wavelength
Put the value into the formula
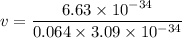
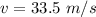
Hence, The speed is 33.5 m/s.