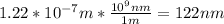Answer:
a) 10.2 eV
b) 122 nm
Step-by-step explanation:
a) First we must obtain the energy for each of the states, which is given by the following formula:
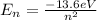
So, we have:
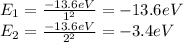
Now we find the energy that the electron loses when it falls from state 2 to state 1, this is the energy carried away by the emitted photon.
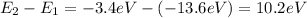
b) Using the Planck – Einstein relation, we can calculate the wavelength of the photon:
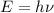
Where E is the photon energy, h the Planck constant and
 the frequency.
the frequency.
Recall that
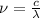 , Rewriting for
, Rewriting for
 :
:
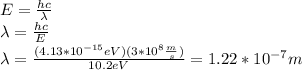
Recall that
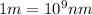 , So:
, So: