Answer:
it move with velocity 1571 m/s
Step-by-step explanation:
given data
wavelength λ = 4.63 ×
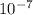 m
m
to find out
how fast is it moving
solution
we will use here de Broglie wavelength equation
that is
wavelength λ =
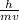 ..........1
..........1
here h is planck constant = 6.626068 ×
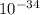
and m is mass of electron i.e = 9.10938188 ×

and v is velocity
put all value we find velocity in equation 1
wavelength λ =
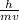
v =
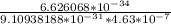
v = 1571.035464
so it move with velocity 1571 m/s