Answer:
(a). The wavelength of photon is 914 A.
(b). The temperature of the black body whose spectrum peaks at wavelength is 31706.78 K.
Step-by-step explanation:
Given that,
Ionization energy = 13.6 eV
(a). We need to calculate the wavelength
Using formula of wavelength
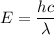
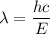
Where, h = Planck constant
c = speed of light
E = energy
Put the value into the formula
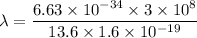
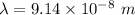
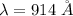
The wavelength of photon is 914 A.
(b). We need to calculate the temperature of the black body whose spectrum peaks at wavelength
Using Wien's displacement law
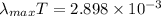
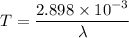
Put the value of wavelength
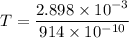
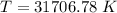
The temperature of the black body whose spectrum peaks at wavelength is 31706.78 K.
Hence, This is the required solution.