Answer:
r=0.228m
Step-by-step explanation:
The equation that defines the states of a gas according to its thermodynamic properties is given by the general equation of ideal gases
PV=nRT
where
P=pressure =5bar=500.000Pa
V=volume
n=moles=10
R = universal constant for ideal gases = 8.31J / (K.mol)
T=temperature=80F=299.8K
solvig For V
V=(nRT)/P
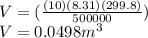
we know that the volume of a sphere is
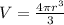
solving for r
![r=\sqrt[3]{ (3 V)/(4\pi ) }](https://img.qammunity.org/2020/formulas/engineering/college/56z9kw433w3jub9tyq98d11s91i8pcypyv.png)
solving
![r=\sqrt[3]{ (3 (0.049))/(4\pi ) }\\r=0.228m](https://img.qammunity.org/2020/formulas/engineering/college/1bnwrb5v2tadlbpl1vphsmsecgnyzg289v.png)