Answer:
3865.74 J/s
Explanation:
mass of ice, m = 1 ton = 1000 kg
time , t = 24 hours
latent heat of fusion of ice, L = 334000 J/kg
Heat required to melt, H = m x L
where, m is the mass of ice and L be the latent heat of fusion
So, H = 1000 x 334000 = 334 xx 10^6 J
Rate of heat transfer = heat / time =
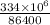
Rate of heat transfer = 3865.74 J/s
thus, the rate of heat transfer is 3865.74 J/s.