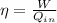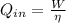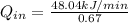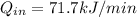Answer:
The rate of heat supplied to the engine is 71.7 kJ/min
Step-by-step explanation:
Data
Engine hot temperature,
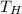 = 900 K
= 900 K
Engine cold temperature,
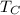 = 300 K
= 300 K
Refrigerator cold temperature,
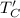 = -15 C + 273 = 258 K
= -15 C + 273 = 258 K
Refrigerator hot temperature,
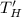 = 300 K
= 300 K
Heat removed by refrigerator,
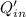 = 295 kJ/min
= 295 kJ/min
Rate of heat supplied to the heat engine,
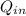 = ? kJ/min
= ? kJ/min
See figure
From Carnot refrigerator coefficient of performance definition
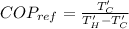
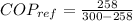
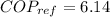
Refrigerator coefficient of performance is defined as
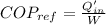
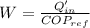
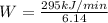
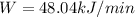
Carnot engine efficiency is expressed as
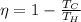


Engine efficiency is defined as