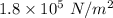Answer:
The gauge pressure is
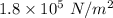
Step-by-step explanation:
Given that,
Gauge pressure of car tires
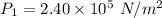
Temperature
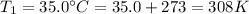
Dropped temperature
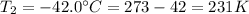
We need to calculate the gauge pressure P₂
Using relation pressure and temperature
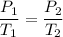
Put the value into the formula
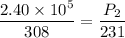
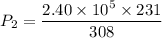
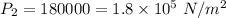
Hence, The gauge pressure is