Answer:
ΔT = 258°C
Step-by-step explanation:
mass of bullet, m = 0.07 kg
velocity of bullet, v = 257 m/s
According to the energy conservation law, the kinetic energy of bullet is totally converted into form of heat energy.
let ΔT be the rise in temperature of the bullet, c be the specific heat of lead.
c = 0.128 J / g°C = 128 J/kg°C
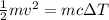
By substituting the values
0.5 x 0.07 x 257 x 257 = 0.07 x 128 x ΔT
ΔT = 258°C