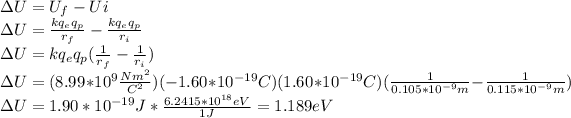Answer:
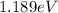
Step-by-step explanation:
The electric potential energy is the potential energy that results from the Coulomb force and is associated with the configuration of two or more charges. For an electron in the presence of an electric field produced by a proton, the electric potential energy is defined as:
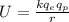
where
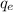 is the electron charge,
is the electron charge,
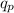 is the proton charge, r is the separation distance between the charges and k is the coulomb constant.
is the proton charge, r is the separation distance between the charges and k is the coulomb constant.
Knowing this, we can calculate how much electric potential energy was lost: